Across pharma and biotech, teams are being asked to deliver the same trials, timelines, and compliance standards with fewer internal resources.
What changed was not the workload.
What changed was access to experienced people who know how to execute.
We consistently hear the same thing:
“It feels like there’s still just as much work to get done; but nobody left to do it.”
What We’re Hearing from Clinical and Technology Leaders
In conversations with clinical operations, supply chain, and IT leaders, a consistent pattern keeps emerging. Teams are smaller than they were a year ago, yet expectations around timelines, quality, and compliance have not moved. On paper, studies are still progressing. In practice, the margin for error feels thinner.
What many leaders describe isn’t chaos; it’s quiet strain that results from industry-wide layoffs.
Work that used to sit with experienced specialists now gets redistributed across already-full calendars. Senior people step in to keep things moving, not because it’s the best use of their time, but because there’s no one else left to do it.
The work didn’t go away, the people who used to absorb it did.
Real-World Situations We See Every Week
We recently spoke with a clinical operations leader who explained that UAT scripts still had to be written, tested, and approved after a reduction in force. The difference wasn’t whether the work happened, but when. What used to be planned work became nights and weekends.
A supply chain team shared a similar story. Forecasting decisions were being made by smart, capable people who simply had not lived inside RTSM systems before. Nothing was “wrong,” yet risk increased because experience had been removed from the process.
An IT director told us that progress slowed even though everyone stayed busy. Time was spent re-explaining systems and filling gaps rather than improving them.
One program lead summarized it best: “We’re not under-resourced; we’re under-supported.”
The Hidden Cost of Fewer eClinical SMEs
When experienced eClinical subject matter experts disappear, organizations rarely fail outright. Instead, small frictions accumulate. Decisions take longer. Risks surface later than they should. Rework quietly increases. Leaders spend more time executing and less time leading.
Over time, confidence erodes before metrics do. Teams sense that they are operating closer to the edge, even if dashboards still look green.
This is rarely a performance issue.
It’s almost always a bandwidth and expertise issue.
A Different Way to Think About Filling the Gap
Most organizations don’t actually need to hire more full-time staff. What they need is access to experienced eClinical SMEs at the moment the work exists.
Not long-term consulting.
Not permanent headcount.
Just experienced people, available part-time and hourly, who already know the systems, the workflows, and the risks.
Support that flexes with study demand rather than fixed org charts.
How BC Consulting Supports eClinical SME Gaps
BC Consulting provides on-demand eClinical subject matter experts who integrate directly into your teams. Our SMEs bring deep, practical experience across IRT and RTSM, eCOA and ePRO, UAT execution, clinical supply operations, and system design and testing.
They step in where work has piled up, where risk is increasing, or where internal experts are stretched too thin. The goal is not to replace your team, but to stabilize execution, reduce pressure, and restore momentum without adding permanent roles.
When This Model Tends to Work Best
This approach is especially effective after reorganizations or layoffs, during high-execution study phases, or when hiring is frozen but timelines are not. It works when internal experts are spread across too many studies and critical work still needs experienced hands.

eClinical experts are ready to help
Our fractional eClinical SMEs plug into your team to improve the performance, oversight and usability of your IRT/RTSM and other eClinical systems.
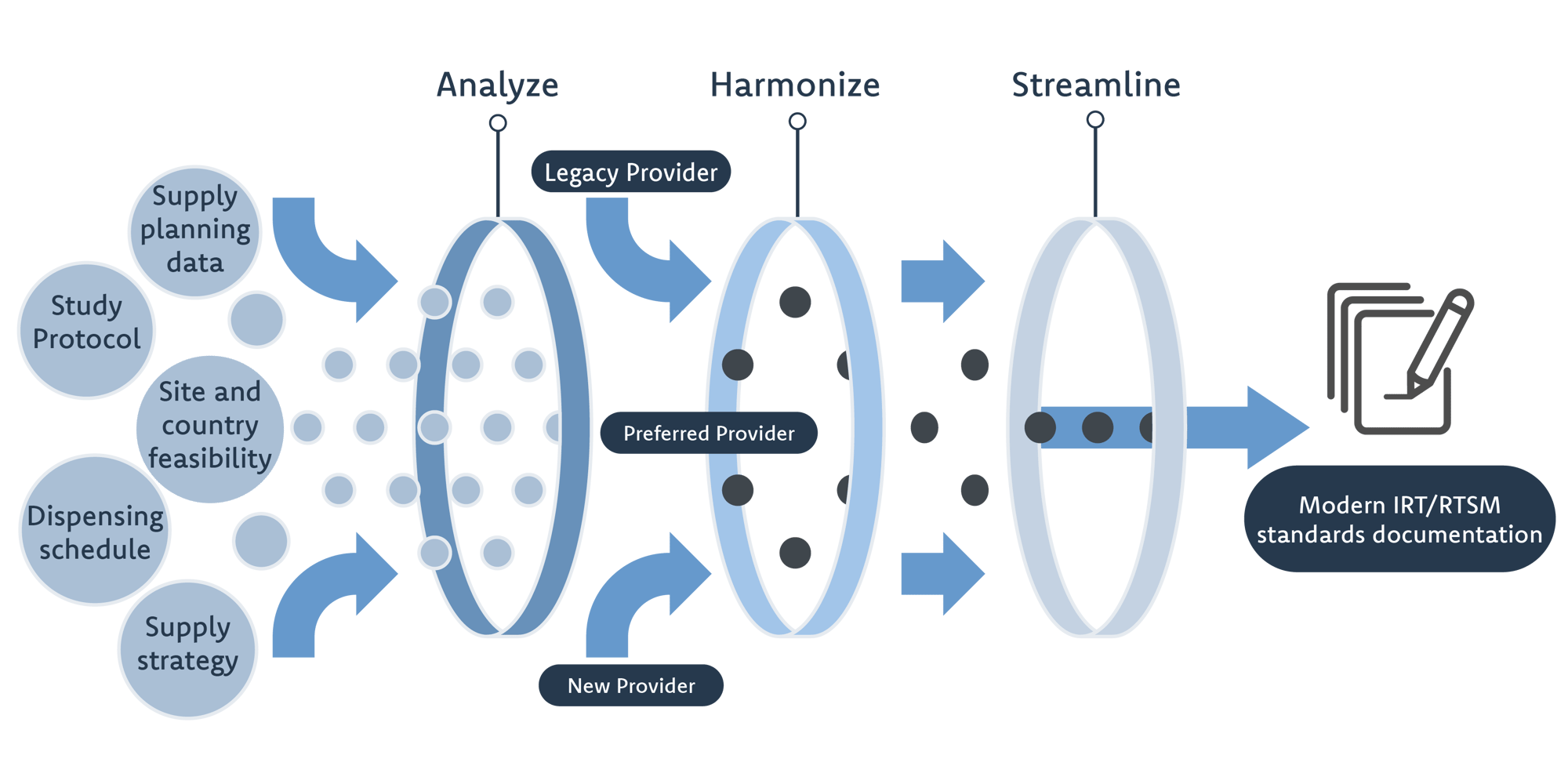
- We conduct discovery and gap analyses across your critical studies to identify operational and compliance risks, and develop study‑level risk matrices.
- We map and harmonise user acceptance testing (UAT) workflows, eliminating redundancies and designing scalable frameworks tailored to your organisation.
- We train super‑users and study teams, support vendor oversight, and provide real‑time advisory during implementation so that new processes stick.
Whether you need a short‑term assessment or ongoing guidance, our experts deliver structured analysis, strategic input and hands‑on support to keep your trials on track.
IRT Standards are evolving
Get expert support on managing the new world of IRT/RTSM standards. Our team is supporting mid-sized and large pharma companies who find themselves with more and more work - but less and less time.
We offer flex resources to help manage:
- Discovery and gap analyses to assess your current SOPs, specifications and vendor flows.
- Risk matrices and process harmonisation to identify pain points and align different vendor workflows.
- Mapping SOPs to IRT workflows and templating UAT scripts so execution is consistent and scalable.
- Implementing industry and regulatory standards across your documents and processes for audit‑ready compliance.
- Training and change management—we train teams on new processes, guide study teams through specification configuration and system changes, and build the soft‑skills needed for risk assessment.
Curious if BC Consulting is the right fit for you?
Our eClinical experts each individually have decades of experience working for both Pharma and Supplier side teams. If you hae other questions about our services, contact us to setup a short call.
Fractional SMEs are seasoned consultants who join your team part‑time or project‑based, providing the same knowledge and guidance as a full‑time hire without the long‑term overhead. You get access to their expertise when you need it and can scale the engagement up or down as your studies or business demands change.
We support every step of the vendor lifecycle—from drafting RFIs/RFPs and evaluating proposals to overseeing implementation and conducting ongoing performance reviews. Our experts know the IRT/RTSM and eCOA/ePRO landscape and can help you choose the right technology partner, mitigate risk and ensure your vendors meet regulatory and operational requirements.
We partner with clinical trial sponsors (pharmaceutical, biotech and medical device companies) as well as eClinical solution and service providers. Our consultants have deep experience across IRT/RTSM, eCOA/ePRO, CTMS and other eClinical technologies, so we understand the needs of both buyers and sellers in this space.
We’re flexible. Most clients choose a fractional or project‑based contract that defines the scope of work and a monthly or project fee. For smaller engagements or ad‑hoc advisory, we can work on an hourly basis. If you’re looking for a longer‑term functional service provider (FSP) arrangement, we can staff dedicated resources to act as an extension of your team for a fixed period. We’ll discuss your needs during the discovery call and recommend the structure that makes the most sense for your goals and budget.
BC Consulting’s eClinical SMEs can step in wherever you need them. Examples of the tasks we routinely handle include:
-
User Acceptance Testing (UAT) – authoring UAT scripts, executing test cases, capturing results and ensuring that issues are resolved before go‑live.
-
Documentation and process management – reviewing and approving specification documents (e.g. IRT functional specifications), writing and updating standard operating procedures (SOPs) and work instructions, and creating templates to standardise processes.
-
System and risk assessments – conducting critical system reviews to assess compliance with 21 CFR Part 11 and other regulations, performing gap analyses across studies and systems, and developing risk matrices to highlight potential issues.
-
Standards implementation – evaluating current practices against industry and regulatory standards, recommending improvements, and implementing harmonised workflows and checklists.
-
Vendor and study oversight – supporting RFI/RFP processes, evaluating vendor proposals, overseeing configuration and change control, and providing day‑to‑day advisory support to study teams.
-
Training and change management – training super users and end‑users on new processes, developing training materials, and guiding adoption through pilot studies.
Whether you need help with a single deliverable or a more comprehensive process redesign, our team can provide targeted, hands‑on support.
What type of companies can we support?

Lorem ipsum dolor sit amet
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Suspendisse varius enim in eros elementum tristique. Duis cursus, mi quis viverra ornare, eros dolor interdum nulla, ut commodo diam libero vitae erat. Aenean faucibus nibh et justo cursus id rutrum lorem imperdiet. Nunc ut sem vitae risus tristique posuere.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Suspendisse varius enim in eros elementum tristique.

Full Name
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Suspendisse varius enim in eros elementum tristique.

Full Name
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Suspendisse varius enim in eros elementum tristique.

Full Name
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Suspendisse varius enim in eros elementum tristique.

Full Name
Lorem ipsum dolor sit amet, consectetur adipiscing elit.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Suspendisse varius enim in eros elementum tristique.
Our Service
Setup a phone call or send us an email. Our customer engagement process is transparent, and non-salesy.
Contact Us
You can use this form to schedule a call that fits your schedule.